Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 23.06.2019 09:00, aaronroberson4940
Weight is a measure of: inertia force matter mass
Answers: 1
You know the right answer?
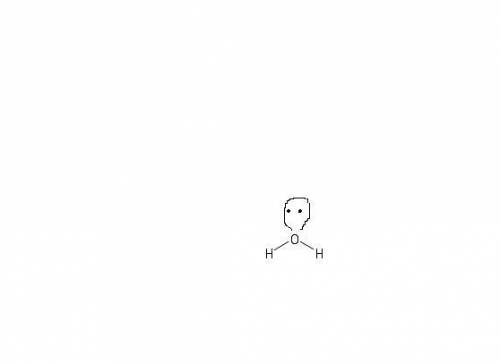
How does a lone pair contribute to molecular shape? a. it is too small to affect the molecule's sha...
Questions in other subjects:


Mathematics, 17.12.2020 19:40


Mathematics, 17.12.2020 19:40

English, 17.12.2020 19:40

English, 17.12.2020 19:40

History, 17.12.2020 19:40


Biology, 17.12.2020 19:40

Mathematics, 17.12.2020 19:40