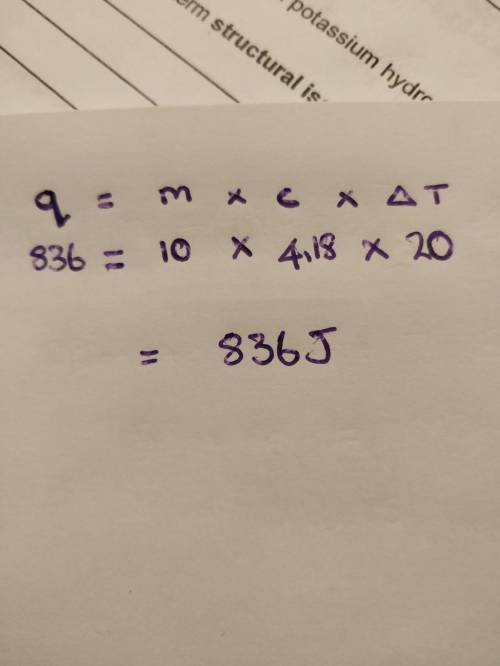Chemistry, 19.07.2019 06:00 johnkings140
What amount of heat is required to raise the temperature of 10.0 g of water from 15.0°c to 35.0°c? the specific heat of water is 4.18 j/g•°c. answer with 3 significant figures.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, nique0808
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 21.06.2019 20:30, dwighthibbert56
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 21.06.2019 22:30, monnn91351
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 14:20, montanolumpuy
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d. lytic
Answers: 1
You know the right answer?
What amount of heat is required to raise the temperature of 10.0 g of water from 15.0°c to 35.0°c?...
Questions in other subjects:

Social Studies, 21.04.2021 03:20

Mathematics, 21.04.2021 03:20



Mathematics, 21.04.2021 03:20

Mathematics, 21.04.2021 03:20

Mathematics, 21.04.2021 03:20