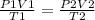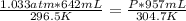Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1


Chemistry, 23.06.2019 19:00, hannahbaak
In which of the following is concentration expressed in percent by volume
Answers: 2
You know the right answer?
A642 ml sample of oxygen gas at 23.5°c and 795 mm hg, is heated to 31.7°c and the volume of the gas...
Questions in other subjects:

Social Studies, 08.10.2019 11:20

Computers and Technology, 08.10.2019 11:20

Computers and Technology, 08.10.2019 11:20


Business, 08.10.2019 11:20



English, 08.10.2019 11:20


Mathematics, 08.10.2019 11:30