Chemistry, 19.07.2019 12:30 cruzhazeL999
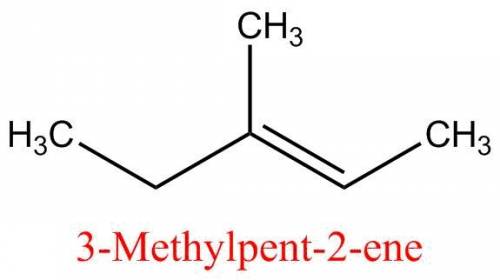
Give the name of the following molecule. a compound with a total of six carbons with a double bond and the rest single bonds. there is a chain of five carbons across the bottom with a double bond between the third and fourth carbons when counting form left to right. there in a methyl group branching off of the middle carbon.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, jude3412
In an effort to address concerns about global warming, a power plant in portland, oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1



Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2
You know the right answer?
Give the name of the following molecule. a compound with a total of six carbons with a double bond a...
Questions in other subjects:




History, 21.05.2020 21:10


Spanish, 21.05.2020 21:10

Mathematics, 21.05.2020 21:10

Mathematics, 21.05.2020 21:10


Mathematics, 21.05.2020 21:10