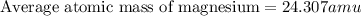Chemistry, 19.07.2019 19:00 erikagibson3414
The naturally occurring isotopes of magnesium are magnesium-24. magnesium-25, and magnesium-26. magnesium-24 has an abundance of 78.994% and a mass of 23.985 amu. magnesium-25 has an abundance of 10.001% and a mass of 24.986 amu. magnesium-26 has an abundance of 11.013% and a mass of 25.983 amu. calculate the average atomic mass of magnesium.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 02:00, sakria2002
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
The naturally occurring isotopes of magnesium are magnesium-24. magnesium-25, and magnesium-26. magn...
Questions in other subjects:


Mathematics, 20.09.2019 12:30

History, 20.09.2019 12:30


Business, 20.09.2019 12:30

History, 20.09.2019 12:30

Mathematics, 20.09.2019 12:30

English, 20.09.2019 12:30


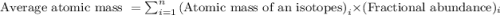 .....(1)
.....(1)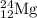 isotope:
isotope: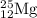 isotope:
isotope: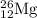 isotope:
isotope:![\text{Average atomic mass of magnesium}=[(23.985\times 0.78994)+(24.986\times 0.10001)+(25.983\times 0.11013)]](/tpl/images/0108/8899/cfd57.png)