
Chemistry, 20.07.2019 00:00 jeffcarpenter
Atritium nucleus is formed by combining two neutrons and a proton. the mass of this nucleus is 9.106 × 10–3 universal mass unit less than the combined mass of the particles from which it is formed. approximately how much energy is released when this nucleus is formed.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:40, timmonskids6027
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1


Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
Atritium nucleus is formed by combining two neutrons and a proton. the mass of this nucleus is 9.106...
Questions in other subjects:

Social Studies, 29.03.2021 19:20

English, 29.03.2021 19:20





Mathematics, 29.03.2021 19:20

German, 29.03.2021 19:20

Mathematics, 29.03.2021 19:20

Mathematics, 29.03.2021 19:20

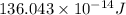



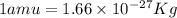

![E=[(9.106\times 10^{-3})\times (1.66\times 10^{-27})Kg]\times (3\times 10^8m/s)^2](/tpl/images/0109/7110/13e90.png)



