
Chemistry, 20.07.2019 00:30 xcncxgnfxg6487
The vapor pressure of pure ethanol at 60 °c is 0.459 atm. raoult's law predicts that asolution prepared by dissolving 10.0 mmol naphthalene (nonvolatile) in 90.0 mmol ethanol willhave a vapor pressure of atm.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 23:00, lulprettyb
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 22.06.2019 23:00, autumperry3599
What is the chemical formula for dihydrogen monoxide
Answers: 2

Chemistry, 22.06.2019 23:00, genyjoannerubiera
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
The vapor pressure of pure ethanol at 60 °c is 0.459 atm. raoult's law predicts that asolution prepa...
Questions in other subjects:

Mathematics, 05.07.2019 14:00

Mathematics, 05.07.2019 14:00

History, 05.07.2019 14:00

Social Studies, 05.07.2019 14:00





Social Studies, 05.07.2019 14:00

Social Studies, 05.07.2019 14:00

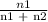 =
= 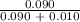 = 0.9
= 0.9

