

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1



Chemistry, 23.06.2019 07:30, mazielynn84
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes or no?
Answers: 1
You know the right answer?
Cl2(g) + 2nabr(aq) 2nacl(aq) + br2(l) the reaction given above is a single replacement reaction beca...
Questions in other subjects:




History, 14.04.2020 04:57


History, 14.04.2020 04:57





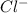 in
in  and
and  in
in  donates electrons to form
donates electrons to form  .
.


