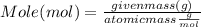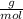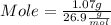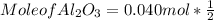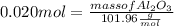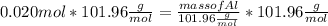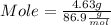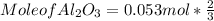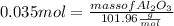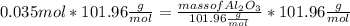Chemistry, 20.07.2019 07:30 awkwardkid0123
What mass of aluminum oxide is produced from the reaction of 4.63 g of manganese dioxide and 1.07 g of al? 3mno2 (s) + 4al(s) -> 3mn (s) + 2al2o3 (s)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, shanicar33500
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 13:30, ayoismeisalex
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
What mass of aluminum oxide is produced from the reaction of 4.63 g of manganese dioxide and 1.07 g...
Questions in other subjects:

Mathematics, 23.06.2020 23:01

Biology, 23.06.2020 23:01





Mathematics, 23.06.2020 23:01

History, 23.06.2020 23:01

Social Studies, 23.06.2020 23:01

Mathematics, 23.06.2020 23:01