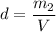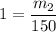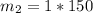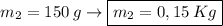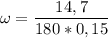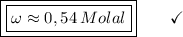Chemistry, 20.07.2019 12:00 Ruthsybel9754
What is the molality of a solution made by dissolving 14.7 g of c6h12o6 into 150.0 ml of water? assume the density of water is 1.00 g/ml?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3



Chemistry, 23.06.2019 12:30, bryantjorell
An atom holds 7 electrons. use orbital notation to model the probable location of its electrons. an atom hold 22 electrons. use orbital notation to model the probable location of its electrons. an atom holds 17 electrons. use orbital notation to model the probable location of its electrons.
Answers: 1
You know the right answer?
What is the molality of a solution made by dissolving 14.7 g of c6h12o6 into 150.0 ml of water? ass...
Questions in other subjects:

English, 11.10.2020 20:01

English, 11.10.2020 20:01

Mathematics, 11.10.2020 20:01


Mathematics, 11.10.2020 20:01

English, 11.10.2020 20:01


History, 11.10.2020 20:01


Mathematics, 11.10.2020 20:01