
Chemistry, 20.07.2019 13:30 FireStorm7327
What is the value for δs°reaction for the following reaction, given the standard entropy values c6h12o6(s) + 6o2(g) —-> 6co2(g) + 6h2o(l) +131 j/k -131 j/k +262j/k -262 j/k +417j/k

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hunterthompson2
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2


Chemistry, 22.06.2019 04:20, lindseysmith9522
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2
You know the right answer?
What is the value for δs°reaction for the following reaction, given the standard entropy values c6h1...
Questions in other subjects:

English, 04.01.2021 20:20



Chemistry, 04.01.2021 20:20



Health, 04.01.2021 20:20



English, 04.01.2021 20:20

 = 212.1 J/K.mol
= 212.1 J/K.mol = 205.0 J/K.mol
= 205.0 J/K.mol = 213.6 J/K.mol
= 213.6 J/K.mol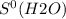 = 69.9 J/K.mol
= 69.9 J/K.mol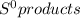 - ∑
- ∑


