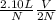Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:00, cami30031cami3003
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
If the number of moles of a gas initially contained in a 2.10 l vessel is doubled, what is the final...
Questions in other subjects:


Health, 10.09.2019 06:20




Chemistry, 10.09.2019 06:20


English, 10.09.2019 06:20

Mathematics, 10.09.2019 06:20

Computers and Technology, 10.09.2019 06:20