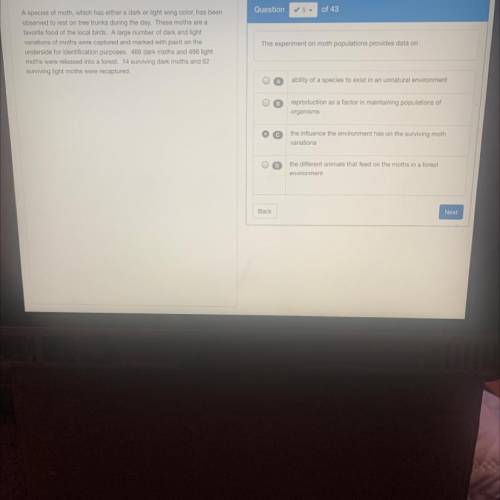
PLZX HELLPPP AH
...

Answers: 1


Other questions on the subject: Biology

Biology, 22.06.2019 08:40, keilyjaramillo2870
What best explains whether bromine (br) or neon (ne) is more likely to form a covalent bond? bromine forms covalent bonds because it has seven valence electrons, but neon has eight valence electrons and already fulfills the octet rule. bromine forms covalent bonds because it has many electron shells, but neon has only two electron shells and is tightly bound to its electrons. neon forms covalent bonds because it can share its valence electrons, but bromine has seven valence electrons and can gain only one more electron. neon forms covalent bonds because it has only two electron shells, but bromine has many electron shells and will lose electrons in order to fulfill the octet rule.
Answers: 3

Biology, 22.06.2019 10:30, isabellemaine
Differentiate renewable and nonrenewable resources
Answers: 1

Biology, 22.06.2019 12:30, Hunter1471
Creating new things to solve problems and improve life depends on the close interaction of which two fields?
Answers: 2

Biology, 22.06.2019 14:00, itsmsjojobabyy5716
Which diagram best represents the chromosomes that would be found in a two new skin cells produced as a result of this process?
Answers: 1
You know the right answer?
Questions in other subjects:

Geography, 04.07.2019 08:50

History, 04.07.2019 08:50


Computers and Technology, 04.07.2019 08:50



Mathematics, 04.07.2019 08:50