
Biology, 17.08.2020 22:01 cameron12502
A. Add to the formula in Interactive Question 3.5 to
show how increasing (CO2) dissolving in water leads
to a lower pH.
b. Use this formula to explain how a lower pH would
affect the [CO32-) in the ocean.
HCO3 SCO32- + H+
c. Assuming a fairly constant (Ca2+] in the ocean, how
would a change in (CO32-) affect the calcification
rate--the production of calcium carbonate
(CaCO3)-by the coral in a reef ecosystem?


Answers: 3


Other questions on the subject: Biology

Biology, 21.06.2019 19:50, hubbabubba0715
Enzymes are proteins that speed up reactions by providing an additional energy source supplying additional molecules for the reaction removing inhibitors that slow down reactions lowering the amount of energy required
Answers: 2



You know the right answer?
A. Add to the formula in Interactive Question 3.5 to
show how increasing (CO2) dissolving in water...
Questions in other subjects:

Biology, 22.01.2021 20:30



Mathematics, 22.01.2021 20:30



Mathematics, 22.01.2021 20:30

English, 22.01.2021 20:30


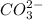 which easily reacts with water to form carbonic acid, thereby increasing water acidity.
which easily reacts with water to form carbonic acid, thereby increasing water acidity.

